Orexin knockout mice ended up utilised for a model of human narcolepsy, which can be characterised by REM sleep dysregulation. The research located that orexin knockout mice show a phenotype comparable to human narcolepsy people Which Modafinil activates orexin-that contains neurons.
Modafinil also increases glutamate’s extracellular levels, a neurotransmitter that activates the NMDA receptors.
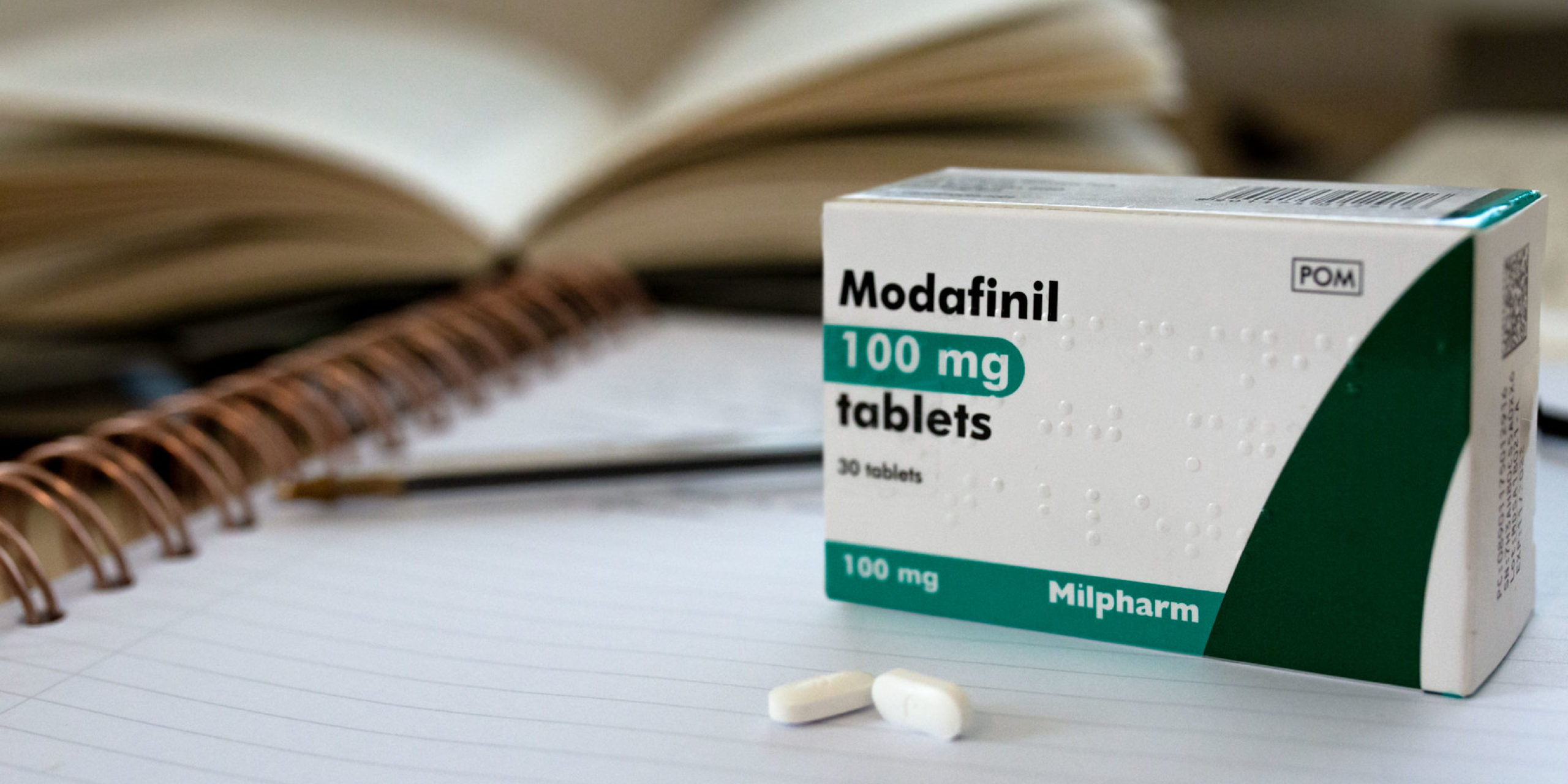
The Modafinil mechanism of action acts as being a dopamine reuptake inhibitor. The consequences of this dopamine cause stimulation with the human Mind and assistance people not sleep off swiftly, keep on being mentally alert, and keep great target.
Evidence shows that depressed individuals with abnormally elevated inflammation are not as likely to respond to classical monoamine reuptake inhibitor therapy (8). Supplied the inflammatory nature of COVID-19 neuropathology, a person could hypothesize an identical pattern amid very long COVID sufferers.
This medication may perhaps trigger accidental overdose and Dying if taken by other adults, youngsters, or pets. Mix any unused medicine that has a compound like cat litter or espresso grounds.
aside from the drug or Lively pharmaceutical ingredient) in such preparations is meant for aside from cure, mitigation, treatment or prevention of any fundamental human condition; or supposed not to have an impact on, the composition or any purpose of a human physique. Again to Citation nine.
one(a)). The probable good quality and safety problems raised by LDPs would usually be evaluated as Portion of the premarket acceptance system, dependant on the evaluation of a broader array of drug development information like certain protection, scientific, and bioavailability or bioequivalence information as proper.[9] Given that compounded drug products that satisfy the ailments of sections 503A and 503B are exempt from premarket approval demands, compounded LDPs would not be subject matter to this sort of assessment dependant on a broader choice of drug advancement knowledge. Thus, compliance with CGMP specifications, by yourself, is unlikely to deliver enough assurance that compounded LDPs can provide product of meant qualities with dependable high quality and constant efficiency. Having said that, FDA is soliciting opinions about irrespective of whether this entry must be additional to just the 503A DDC Record or only the 503B DDC Listing. FDA is aware that specific FDA-authorized liposome drug solutions may have Guidance inside their authorized labeling for particular manipulations. Accordingly, FDA is likewise soliciting feedback about whether the entry for that 503B DDC Listing ought to incorporate any restrictions, for example, such as, to address specific LDPs that an outsourcing facility compounds from FDA-authorized liposome drug solutions. three. Drug Products and solutions Made Utilizing HMEs
Even so, Gerrard et al. suggest that far more operate be carried out on examining possible intracellular mechanisms of Modafinil and finding a point of convergence of its stimulant and neuroprotective consequences.
The initial research identified the MPTP-induced parkinsonism signs or symptoms could be improved with Modafinil 11 months following MPTP administration.
FDA tentatively concludes this proposed rule contains no assortment of data as defined because of the Paperwork Reduction Act of 1995.
This formulation is necessary to ensure that the API has increased solubility, resulting in the desired bioavailability of the drug merchandise. To avoid a detrimental impact on the protection and efficacy of the product or service, the extrudate should have a uniform distribution of API while in the matrix and a managed amount of impurities. It can be crucial for these formulations for being thermally steady in the extrusion system and bodily secure Later on. Uncooked materials variety and Command and component ratios influence many characteristics of the extrudate and, consequently, the ultimate product. If HMEs are certainly not formulated accurately, making an allowance for the concepts mentioned earlier mentioned, it could lead on to sizeable variability in functionality within just and throughout batches, and may effect bioavailability. The drug shipping and delivery mechanism, or perhaps the mechanism by which API is introduced with the HMEs, can even be advanced mainly because it is depending on an item design and style ( e.g.,
In evaluating drug products or groups of drug products with the DDC Lists, the Agency proposes to contemplate these conditions independently and collectively, and to take into consideration the hazards and Gains to people from the compounded drug products or classes of drug products and solutions. The factors are not mutually distinctive. A drug product or group of drug solutions might meet up with a number of of these standards that indicate it offers demonstrable challenges for compounding. FDA proposes to use the same requirements When it comes to drug merchandise or types of drug products for inclusion on either the DDC List for area 503A or perhaps the DDC Listing for section 503B from the FD&C Act, Despite the fact that the appliance of the standards might cause different conclusions for every record.
Additionally, to evaluate and guarantee dependable purity on the drug product, chemical impurities have to be quantitated through different delicate analytical strategies formulated specifically for these impurities.
In the 2nd more information study, they uncovered that Modafinil administration with MPTP was not able to prevent Preliminary locomotor results of MPTP but was capable to revive locomotor action within just two months.